Important Safety Information
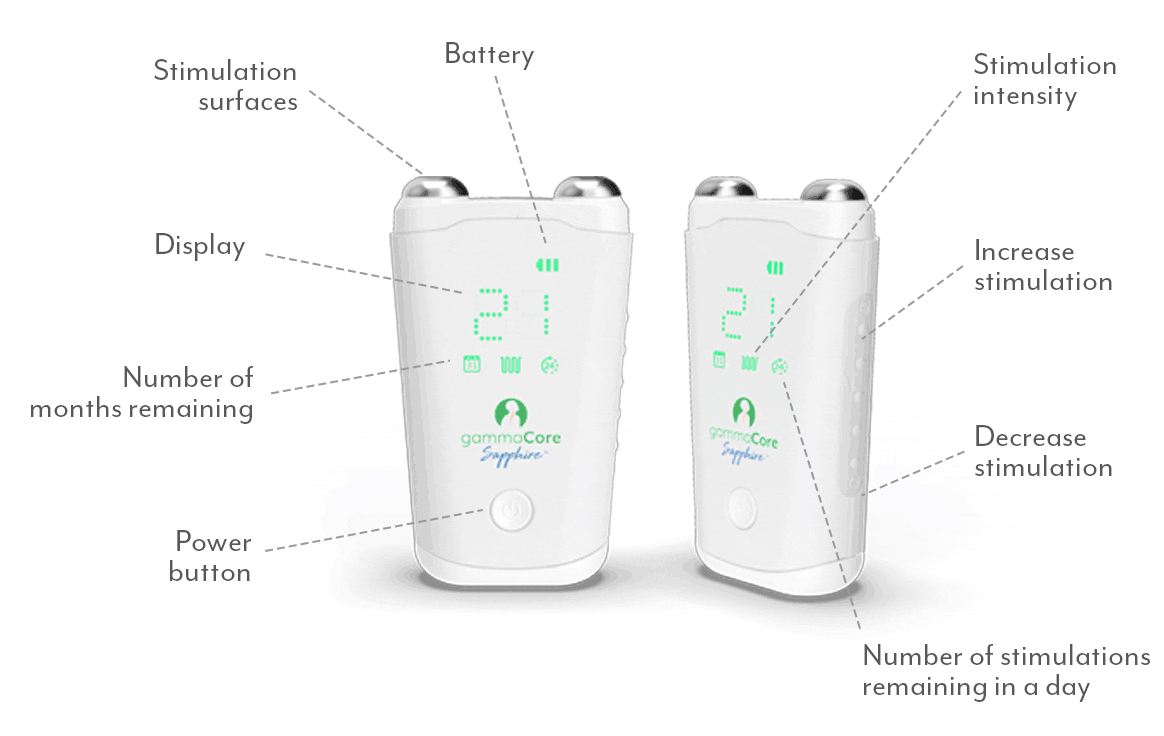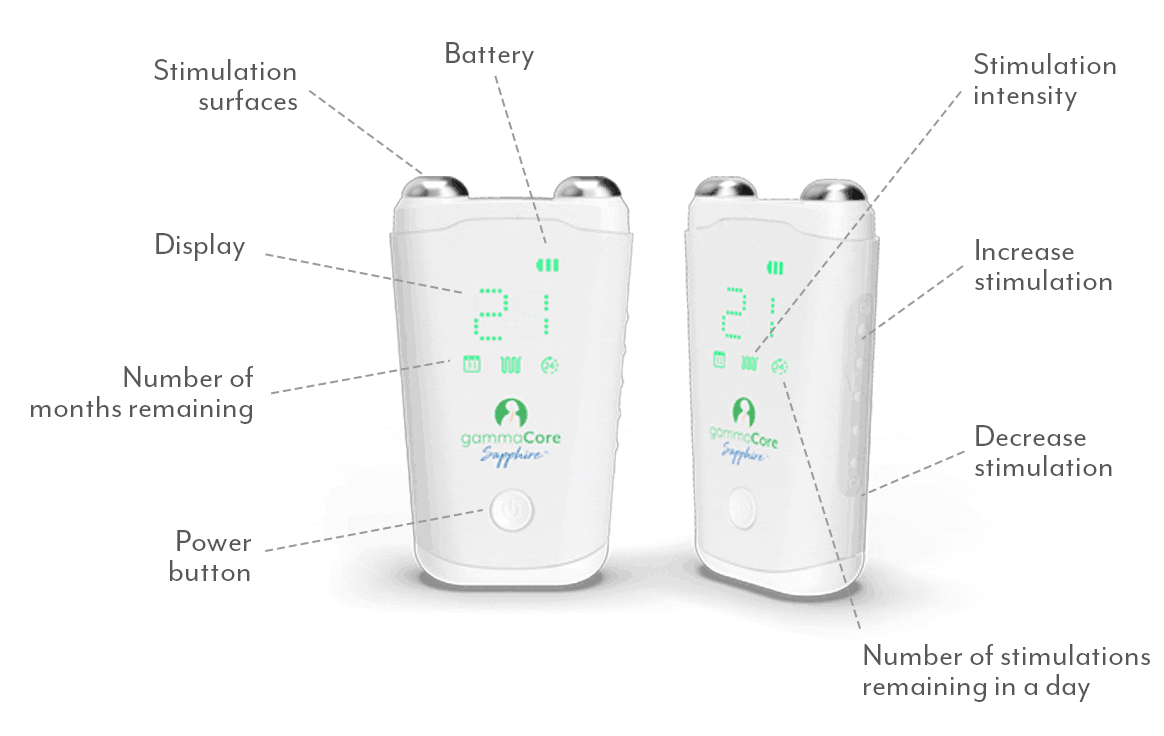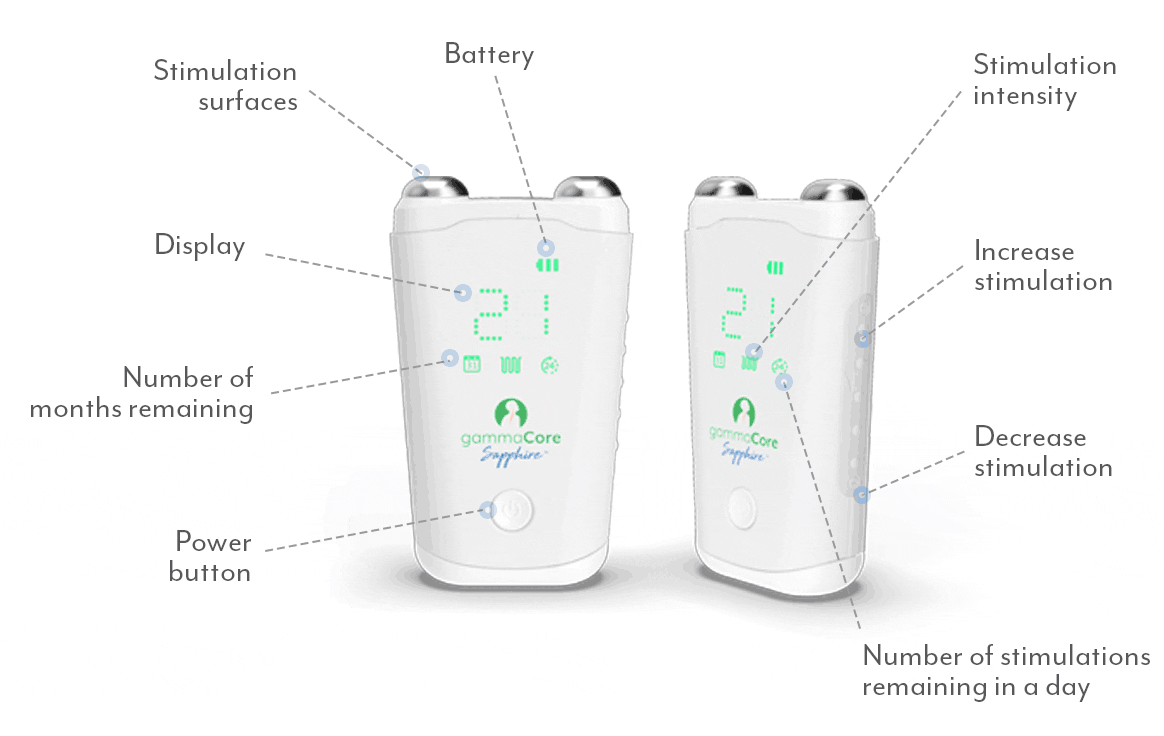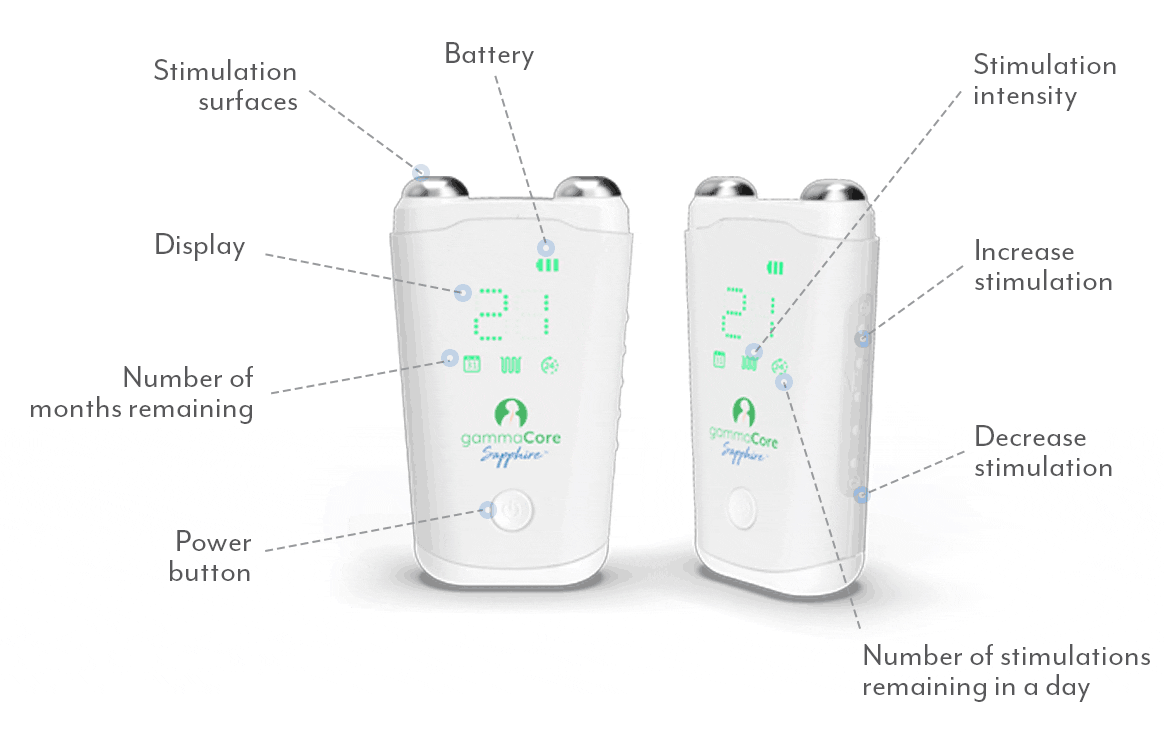
gammaCoreTM (non-invasive vagus nerve stimulator) is intended to provide non-invasive vagus nerve stimulation (nVNS) on the side of the neck. gammaCore is indicated for:
- The preventive treatment of migraine headache in adolescent (age 12 and older) and adult patients.
- The acute treatment of pain associated with migraine headache in adolescent (age 12 and older) and adult patients.
- Adjunctive use for the preventive treatment of cluster headache in adult patients.
- The acute treatment of pain associated with episodic cluster headache in adult patients.
- Treatment of hemicrania continua and paroxysmal hemicrania in adults.
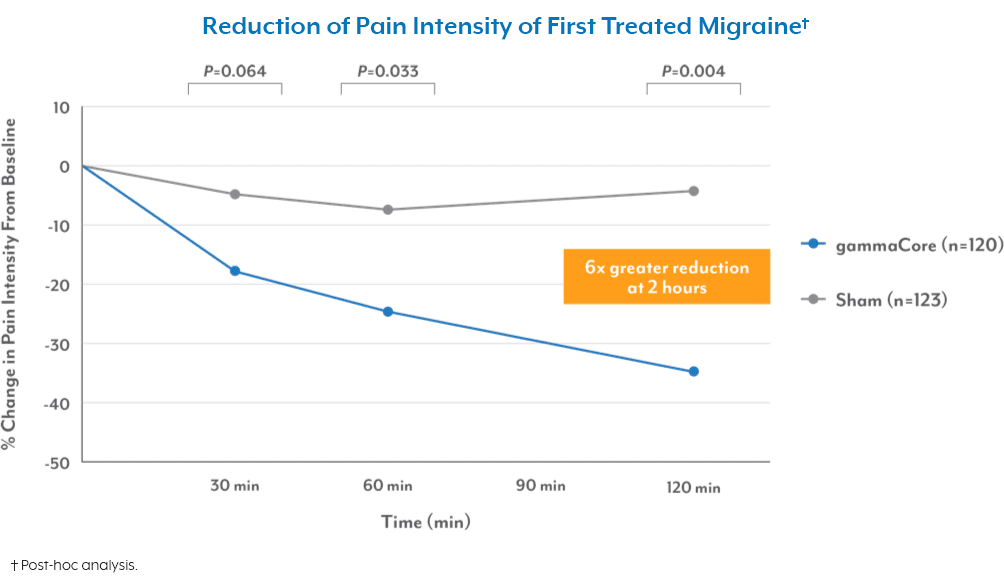
- The effectiveness of gammaCore has not been established in the acute treatment of chronic cluster headache
- gammaCore is contraindicated for patients if they:
- Have an active implantable medical device, such as a pacemaker, hearing aid implant, or any implanted electronic device
- Are using another device at the same time (e.g., TENS Unit, muscle stimulator) or any portable electronic device
- Safety and efficacy of gammaCore have not been evaluated in the following patients:
- Patients diagnosed with narrowing of the arteries (carotid atherosclerosis)
- Patients with a metallic device, such as a stent, bone plate, or bone screw, implanted at or near the neck
- Patients who have had surgery to cut the vagus nerve in the neck (cervical vagotomy)
- Pediatric patients (younger than 12 years)
- Pregnant women
- Patients with clinically significant hypertension, hypotension, bradycardia, or tachycardia